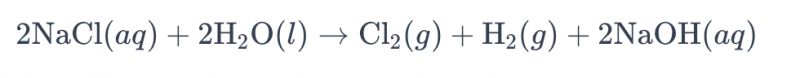ʻO ke kaʻina hana o ka electrolyzing solution brine me ka hoʻohana ʻana i nā electrodes titanium e hana i ka chlorine i kapa ʻia ʻo "electrolysis of brine." Ma kēia kaʻina hana, hoʻohana ʻia nā electrodes titanium e hoʻomaʻamaʻa i ka hopena o ka oxidation o nā ion chloride i loko o ka brine, e alakaʻi ana i ka hana o ke kinoea chlorine. ʻO ka hoʻohālikelike kemika holoʻokoʻa no ka pane ʻana penei:
Ma keia hoohalike, chloride ions i ka oxidation ma ka anode, ka hopena i ka hana ana o ka chlorine kinoea , oiai ka wai molekele i hoemiia ma ka cathode, yielding hydrogen kinoea. Hoʻohui hou, hoʻemi ʻia nā ion hydroxide ma ka anode, hana i ke kinoea hydrogen a me ka sodium hydroxide.
ʻO ke koho ʻana i nā electrodes titanium ma muli o ke kūpaʻa maikaʻi o ka titanium a me ka conductivity, e ʻae iā ia e hoʻokō i ka hopena i ka wā electrolysis me ka ʻole o ka corrosion. Hana kēia i nā electrodes titanium i koho kūpono no ka electrolysis o ka brine.
Pono ka electrolysis o ka wai paʻakai i kahi kumu mana waho e hāʻawi i ka ikehu no ka hopena electrolytic. ʻO kēia kumu mana ka mea maʻamau i ka mana o kēia manawa (DC) no ka mea e pono ai nā hopena electrolytic i kahi kuhikuhi kūlike o ke kahe o kēia manawa, a hiki i kahi mana DC ke hāʻawi i kahi kuhikuhi mau.
I ke kaʻina hana o ka electrolyzing wai paʻakai e hoʻohua i ke kinoea chlorine, hoʻohana mau ʻia kahi mana DC haʻahaʻa haʻahaʻa. ʻO ka volta o ka lako mana e pili ana i nā kūlana hoʻohālikelike kikoʻī a me ka hoʻolālā ʻana i nā mea hana, akā ma waena o 2 a 4 volts. Eia kekahi, ʻo ka ikaika o ka mana o kēia manawa he mea koʻikoʻi nui e pono e hoʻoholo ʻia e pili ana i ka nui o ke keʻena pane a me ka hopena o ka hana i makemake ʻia.
I ka hōʻuluʻulu ʻana, ʻo ke koho ʻana i ka mana no ka electrolysis o ka wai paʻakai e pili ana i nā koi kikoʻī o nā hoʻokolohua a i ʻole nā kaʻina hana ʻoihana e hōʻoia i ka hopena kūpono a me ka loaʻa ʻana o nā huahana i makemake ʻia.
Ka manawa hoʻouna: Jan-16-2024